Organization of production practice “Quality control and standardization of medicines” using distance learning technologies
The production practice “Quality Control and Standardization of Medicines” was completed at the 5th year students of the Faculty of Pharmacy, conducted in the new distance learning format.
To carry out practical training at the Department of Pharmaceutical and Toxicological Chemistry, a lot of organizational work was carried out: an algorithm for remote interaction between the teacher and students was developed, digital communication tools were identified, video clips (38) were recorded for laboratory tasks, the contents of tasks, monitoring forms, etc. were prepared. The content of the assignments for practical training was posted on the AIS Platonus in the module “Assignments”.
Previously, an installation conference was held on the Webex platform, where teachers introduced students to the practice program, assignments, guide-lines for their implementation, diaries, literature necessary to prepare for the up-coming practice, instructed students on all issues of filling out the diary and con-ducting a test based on the results of practice .
The resources and tools of the Google Classroom platform with a user-friendly interface and wide functionality were used as the main tool for conducting production practices in remote mode.
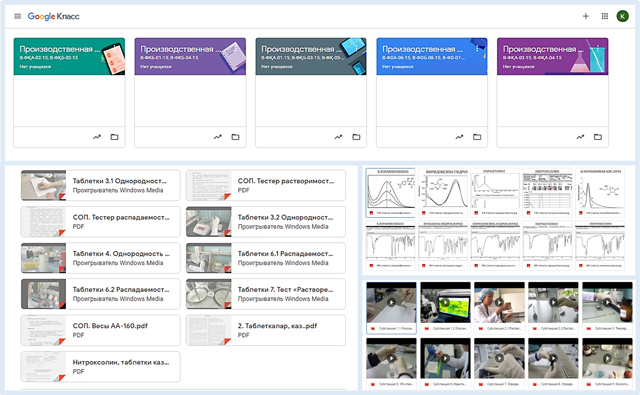
A virtual class page was opened for each study group in Google Classroom, in which practice leaders published tasks on the tabs “Instructions”, “Tasks”, “Monitoring Assessment”, “Users”, etc. In the “Instructions” section, students received detailed instructions for completing tasks on the pharmacopoeial analysis of medicinal substances, tablets, injection solutions. In the "Assignments", students had access to training videos, regulatory documents, orders of the MHRK, OPS for instruments and equipment, teaching materials.
To complete the tasks, trainees studied the materials obtained, video clips on the implementation of a specific section of the pharmacopoeial analysis. For sustained feedback and monitoring of assignments, the practice leader conducted a lesson daily on the Zoom, or Webex platform, students commented on video clips on the work of the pharmacist-analyst in monitoring the quality of drugs in accordance with the requirements of regulatory documents, the procedure on devices and equipment in accordance with OPS.

After a theoretical discussion and a virtual analysis of drugs, students filled out daily diaries in electronic form and sent them to teachers daily for review in Google Classroom. The head of practice had the opportunity to write his com-ments, additions, recommendations in electronic form on the “Comment” tab.
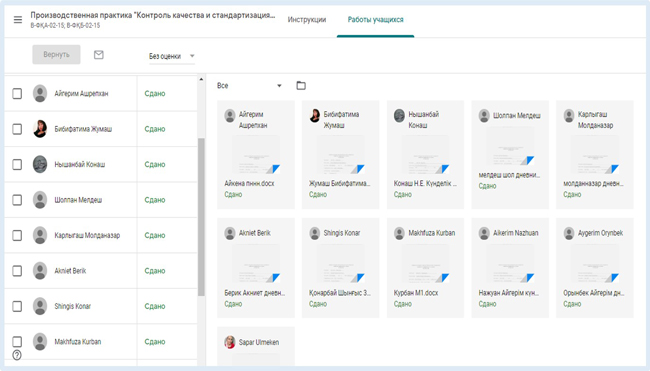
Thus, in Google Classroom, for each trainee, a personal portfolio is formed, consisting of instructions, assignments, a diary, a calendar, deadlines for completing assignments, comments, comments by the practice leader, and work on errors.
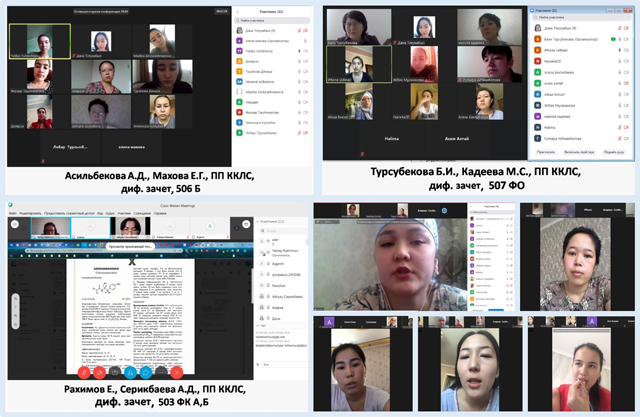
The production practice ended with a differentiated classification, which was carried out on a commission basis using remote technologies on Zoom and Webex platforms.
After completing the practice, a final conference and questionnaires were conducted with students using an electronic questionnaire. Student feedback and processing of questionnaire data showed that students note a high level of training and practice, the ability to obtain sustainable practical skills in a remote format.
Thus, for the organization and conduct of the production practice “Quality Control and Standardization of Medicines” in the remote format, the Department of Pharmaceutical and Toxicological Chemistry provided information and methodological support with the presentation of a complete package of methodological, reference and other materials, as well as counseling students.
 725 views
725 views
