Organization and implementation of industrial practice "quality control and standardization of medicines" using distance learning technologies
Completed industrial practice "Quality control and standardization of medicines" for 5th year students of the Faculty of Pharmacy, held in the format of distance learning.
The bases of industrial practice this year were the Testing Laboratories of the National Center for Expertise of Medicines and Medical Devices of the Committee for Medical and Pharmaceutical Control of the MH RK in the cities of Taraz and Karaganda.
To carry out practical training at the Department of Pharmaceutical and Toxicological Chemistry, a lot of organizational work was carried out: an algorithm for remote interaction between the teacher and students was developed, digital communication tools were identified, videos (40) were recorded on laboratory assignments, the content of assignments, monitoring forms, etc. were prepared. The content of the assignments for the production program was posted on a Google Drive platform and then sent to the students. Students in their personal account through the online.skma.edu.kz website filled out electronic diaries in accordance with the daily practice.
Preliminarily, on the ZOOM platform, an orientation conference was held, at which teachers and leaders from the practice bases Daurbaeva Kh.A., the head of the NCEMMD department in the city of Taraz and the coordinator of the NCEMMD department in the city of Karaganda introduced the students to the internship program, tasks, methodological instructions on their implementation, diaries, literature necessary to prepare for the upcoming practice, instructed students on all issues of filling out a diary and holding a test based on the results of practice.
A virtual class page was opened for each study group in skma.edu.kz, in which practice leaders published assignments on a daily basis in the "Instructions", "Assignments", "Monitoring-Assessment", "Users" tabs, etc. In the "Instructions" section, students received detailed instructions for completing tasks on the pharmacopoeial analysis of medicinal substances, tablets, and injection solutions. In the "Tasks", students had access to training videos, regulatory documents, orders of the MH RK, SOP for devices and equipment, methodological materials.
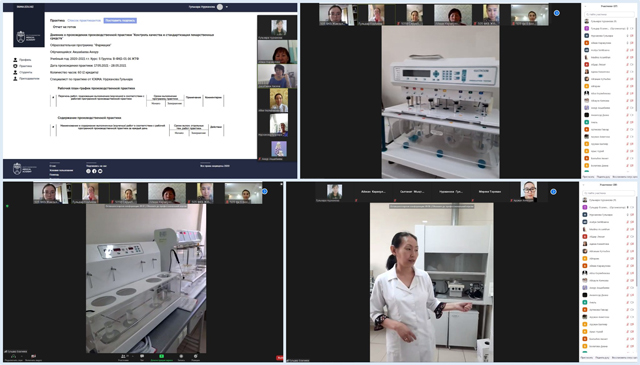
To complete the tasks, the trainees studied the materials received, videos on the implementation of a certain section of the pharmacopoeial analysis. For stable feedback and monitoring of assignments, the head of the practice conducted a daily lesson on the Zoom platform, students commented on videos on the work of a pharmacist-analyst in quality control of medicines in accordance with the requirements of regulatory documents, the procedure for devices and equipment in accordance with the SOP.
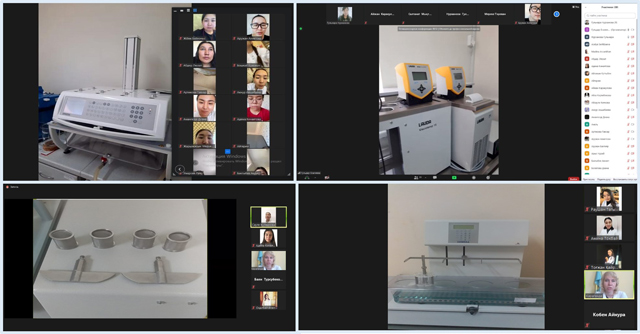
The industrial practice ended with a differentiated exam, which was carried out by commission using remote technologies on the Zoom platform.
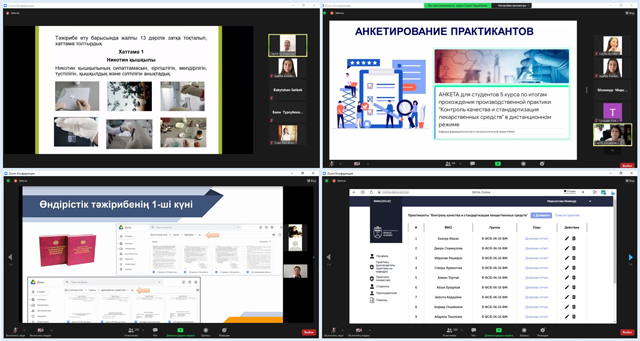
After completing the practice, a final conference and questionnaire were held with the students using an electronic questionnaire. Feedback from students and processing of questionnaire data showed that students note a high level of preparation and practice, the possibility of obtaining sustainable practical skills in a distance format.The heads of the practice bases also noted the high level of preparatory work of the department staff.
Thus, for the organization and conduct of industrial practice "Quality control and standardization of medicines" in a remote format at the Department of Pharmaceutical and Toxicological Chemistry, information and methodological support was provided with the presentation of a full package of methodological, reference and other materials, as well as consulting students.
 944 views
944 views
